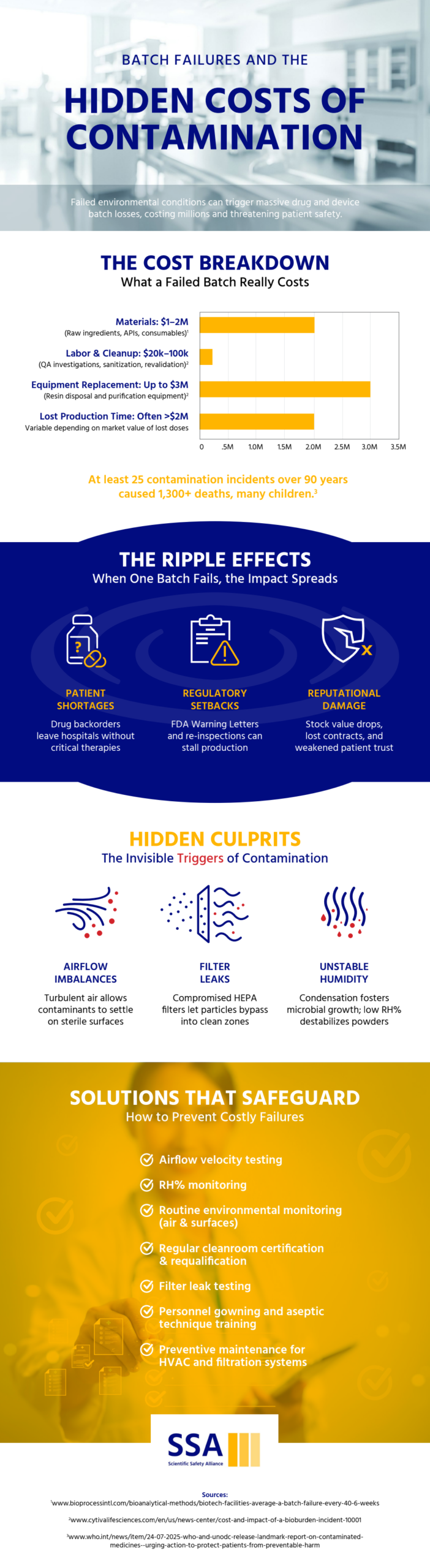
In pharmaceutical and medical device manufacturing, contamination is not simply a technical error. It represents a systems level failure that touches every aspect of operations. While the immediate loss of a production batch may seem like the primary concern, the broader impact extends to financial performance, scheduling stability, and organizational reputation.
The Costly Domino Effect of Remediation
When contamination is identified, the response requires more than basic cleaning. Manufacturers must conduct detailed investigations and implement comprehensive corrective measures. This often includes requalifying cleanrooms, revalidating equipment, and reassessing environmental monitoring processes. Each step contributes to extended downtime, delaying product release and disrupting carefully coordinated production schedules.
Financial consequences escalate quickly. Scrapped materials, repeat sterility testing, and duplicated validation efforts generate expenses that were never included in original budgets. As these unexpected costs accumulate, they can erode profit margins and strain operational resources.
Pressure Across the Supply Chain
The effects of contamination rarely remain confined to a single production area. Even in situations where product safety has not been definitively compromised, manufacturers may need to quarantine batches while conducting additional testing. These precautionary steps, though necessary, can slow throughput and create bottlenecks across the manufacturing pipeline.
Facilities operating near capacity are particularly vulnerable. Delays can result in missed delivery commitments or product shortages. Healthcare providers relying on steady supply may need to seek alternative sources, potentially affecting patient access to essential treatments.
Impact on Equipment and Facility Infrastructure
Intensive decontamination efforts can also take a toll on equipment and facility systems. Strong chemical agents and repeated high temperature sterilization cycles may degrade sensitive components such as filters, tubing, chromatography resins, and other consumables. In many cases, these materials must be replaced entirely to maintain compliance.
Even after production resumes, equipment exposed to contamination events may require additional maintenance or experience shortened service life, adding to ongoing operational costs.
Reputation and Regulatory Consequences
Beyond operational and financial implications, contamination incidents can weaken stakeholder confidence. Even without a formal recall, regulators may increase oversight and require corrective action plans, expanded documentation, or more frequent inspections.
Customers, investors, and partners may interpret contamination as an indicator of deeper quality system weaknesses. Rebuilding trust often requires months of transparent communication, enhanced quality reviews, and demonstrable process improvements.
Addressing Root Causes, Not Just Symptoms
Contamination events rarely stem from a single isolated issue. More often, they reveal underlying vulnerabilities in facility design, environmental controls, or personnel practices. Addressing these weaknesses may involve upgrading cleanroom layouts, improving airflow management, strengthening filtration systems, or reinforcing aseptic techniques through focused training initiatives.
Although these enhancements require investment, they ultimately improve the resilience and compliance of the manufacturing environment.
Prevention as a Strategic Imperative
Effective contamination control extends beyond incident response. It requires proactive planning and an integrated quality framework that aligns facility design, operational processes, and workforce training. Maintaining consistent environmental control and process discipline is essential to reducing risk.
In today’s regulatory landscape, contamination prevention is both a cleanroom compliance obligation and a competitive differentiator. Organizations that prioritize prevention protect not only their operations and financial performance, but also the trust that underpins their role in the healthcare industry.